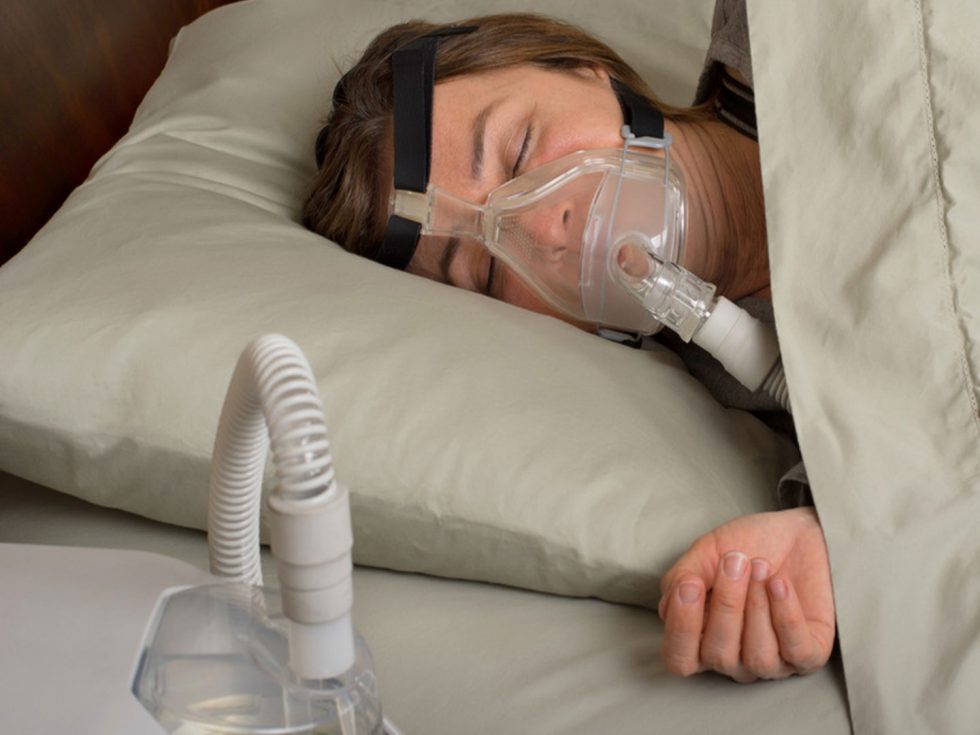
At least 70,000 Belgian sufferers from a condition known as sleep apnea have joined with Dutch counterparts in a legal action against consumer electronics giant Philips that could cost the company billions in damages.
Sleep apnea (from the Greek meaning ‘lack of breath’) is a condition generally caused by a loss of tone in the muscles at the back of the throat.
While the sufferer sleeps, those muscles relax and occlude the passage of air. The subject wakes up partially and resumes breathing, but the problem continues as long as the person sleeps, leading to hundreds or more of such episodes a night.
The result is an extremely disturbed night’s sleep, and the fatigue and ill health that follow.
Nowadays, sleep apnea is usually treated with the help of a machine which – to simplify matters greatly – uses positive air pressure delivered through a face mask to keep the airway open and allow the user to breathe freely.
Not all such machines, which are small enough to sit on a bedside table and are easy to use once the initial strange feeling of wearing a face mask in bed is overcome, are made by Philips, but to date the Dutch company is the only one concerned with legal action.
The extent of the company’s problems cannot be over-estimated. Following multiple visits by the US regulator, the Food & Drug Administration (FDA) to Philips plants, complaints were raised about the use of a noise-dampening material in the casing of the machine. The administration found that particles of polyester-based foam were being delivered by the apparatus directly to the user’s lungs.
Philips replaced the polyester foam with a silicone alternative, but concerns persist. Some machines in the US have been recalled. More importantly from a European point of view, the FDA investigation has provided grist to the mill for plaintiffs in Europe.
The result as it now stands is that Philips has been forced to recall some 3.5 million machines in Europe, and the question of damages has still to be handled in court. Already, since the company first made the problems known in April this year, the share price of what would normally be considered one of the Netherlands’ golden-ticket companies, has plunged by 35%, representing a loss in stock value of some €15 billion.
The problems don’t stop there. This year, since the problem arose, Philips has spent €500 million on its recall action for existing machines, and faces a loss of €600 million on the lost sales of new machines.
For the company, two problems loom. On the one hand, the damage to its reputation could be enormous. Problems with a steam iron are one thing: problems with a device that literally forces air into a person’s lungs while they sleep are on another level.
The company claims it has dealt adequately with claims of problems, by for example replacing its silicone-based foam with polyester. And in any case, the company claims, complaints concerned only 0.03% of all machines in use – a defence, it has to be said, that is risky at the best of times.
And even the most objective observer would have to wonder at other defence mechanisms employed by Philips.
Earlier this year, when the company ultimately started its recall of machines, it explained to users that the degradation of materials could be caused by humidity and warmth – conditions that are surely present in most bedrooms. Philips also pointed the finger for the problems of foam degradation at “cleaning methods that were not approved”.
The legal action before the European courts, involving Dutch and Belgian users of the machines, will keep its eyes keenly focussed on the American court case, based on 31 visits by the FDA to Philips’ production facilities in the US.
In the meantime, another problem looms for Philips on the horizon: according to reports, the FDA has now uncovered evidence that the company was informed about the problems, and did nothing. If that accusation turns out to carry weight, the company could face a flood of lawsuits for negligence.