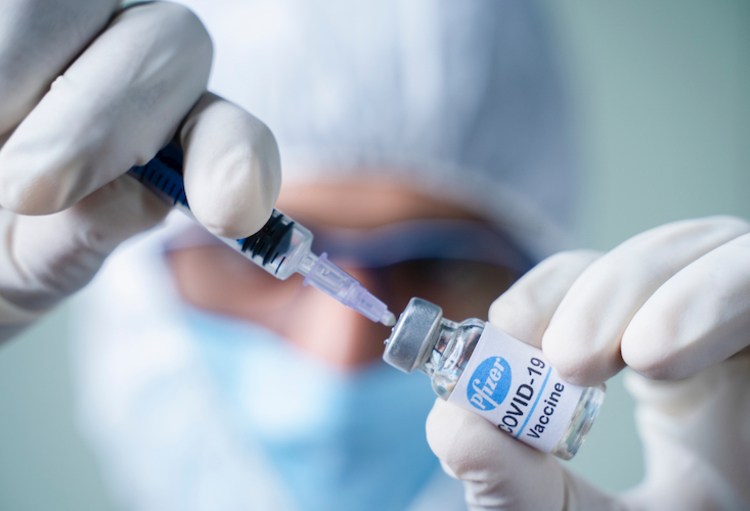
The coronavirus vaccine developed by pharmaceutical companies Pfizer and BioNTech has proven to be 100% effective in phase three clinical trials, Pfizer announced on Wednesday.
The results from the trial, involving 2,260 children in the United States, exceeded those reported in the research on the vaccine in 16-25-year-old participants, a press release stated.
“The initial results we have seen in the adolescent studies suggest that children are particularly well protected by vaccination, which is very encouraging given the trends we have seen in recent weeks regarding the spread of the British variant,” said Ugur Sahin, CEO, and Co-founder of BioNTech.
Today, with @BioNTech_Group, we announced positive topline results in adolescents 12-15 years of age from the Phase 3 Pfizer-BioNTech #COVID19 vaccine study.
— Pfizer Inc. (@pfizer) March 31, 2021
In the trial, 18 cases of Covid-19 were observed in the placebo group, whilst none were identified in the vaccinated group, and side effects were found to be consistent with those observed in participants 16 to 25 years of age.
Related News
- Pfizer to produce 2.5 billion coronavirus vaccines, 500 million more than expected
- EU vaccine strategy: Priority goals not reached by March
- Deaths as a result of coronavirus rise, but hospitalisations continue to drop
The company will now submit the data to the European Medicines Agency (EMA) to request the expansion of the EU Conditional Marketing Authorisation for the vaccine and hopes to start vaccinating this group before the start of the next school year.
“Across the globe, we are longing for a normal life. This is especially true for our children. It is very important to enable them to get back to everyday school life and to meet friends and family while protecting them and their loved ones,” Sahin said in a statement.
All participants in the trial will continue to be monitored for long-term protection and safety for an additional two years after receiving their second dose.
Last week, Pfizer and BioNTech it had administered the first doses in first healthy children between the ages of 6 months to 11 years in a two-dose schedule based on three different age groups within this category.
On Tuesday, the pharmaceutical companies announced they will be producing 2.5 billion coronavirus vaccines this year, 500 million more than the expected 2 billion, which means over one billion people could be fully protected against the virus.
Lauren Walker
The Brussels Times