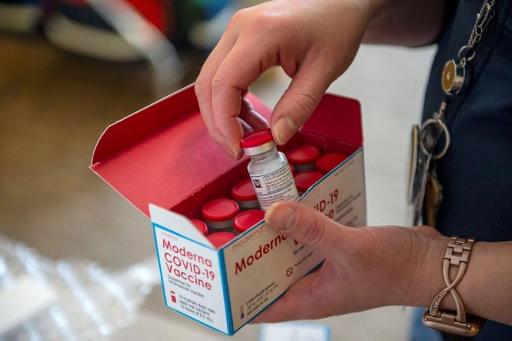
The US-developed coronavirus vaccine Moderna has officially become the second coronavirus vaccine approved for use in the EU after it was officially authorised today.
Taking to Twitter on Wednesday Afternoon, Commission President Ursula von der Leyen announced the news of the second vaccine approved for use in the EU.
We are providing safe & effective #COVID19 vaccines for Europeans.
We have authorised the @moderna_tx vaccine, the 2nd vaccine approved in the EU. Europe has secured so far 2 billion doses of potential vaccines - more than enough for protecting us all #StrongerTogether pic.twitter.com/Ujg4C997fK — Ursula von der Leyen (@vonderleyen) January 6, 2021
This news comes after the European Medicines Agency (EMA) recommended granting conditional marketing authorisation for the vaccine earlier on Wednesday.
“Good news for our efforts to bring more Covid-19 vaccines to Europeans,” tweeted von der Leyen in a reaction to the announcement. “Now we are working at full speed to approve it and make it available in the EU.”
This news makes Moderna the second vaccine that can be used in Europe against the coronavirus after the BioNTech/Pfizer vaccine was granted on 21 December.
By allowing the vaccine, the EU joins the United States, Canada and Israel, who have already granted authorisation.
The Brussels Times