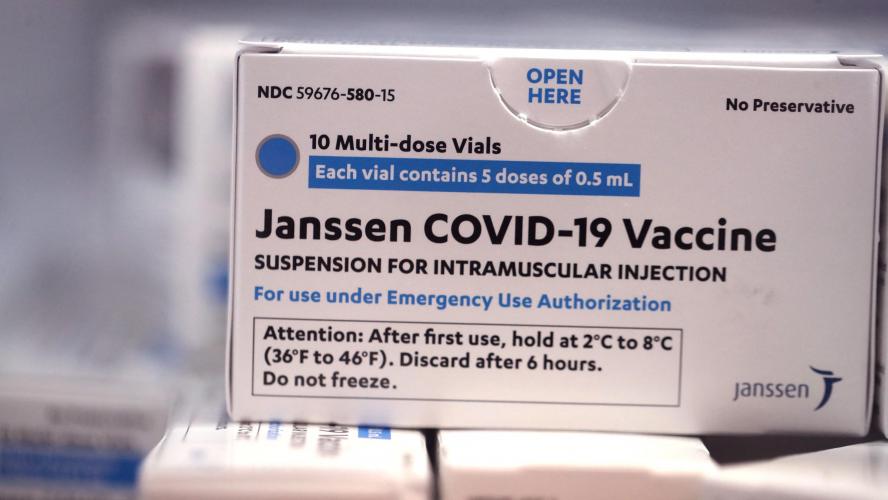
The American pharmaceutical company Johnson & Johnson will "proactively" delay deliveries of its coronavirus vaccine to Europe following reports of rare blood clots after vaccination in the US.
The postponement follows the recommendation from the US health authorities to temporarily pause using the vaccine, after six cases of rare blood clots were reported after vaccination, the company announced in a press release on Tuesday.
"The safety and well-being of the people who use our products is our number one priority," the company said. "We are aware of an extremely rare disorder involving people with blood clots in combination with low platelets in a small number of individuals who have received our Covid-19 vaccine."
On 12 April, over 6.8 million doses of the vaccine have been administered in the US, according to the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC), who issued a joint statement to recommend a vaccination pause "out of an abundance of caution."
Related News
- Two million people have now received first vaccine dose in Belgium
- Janssen vaccine also under EMA scrutiny for four blood clots
- Brussels GPs can now vaccinate patients who can't get to centres
All six cases occurred in women between 18 and 48 years old, with symptoms showing up six to 13 days after vaccination, according to the FDA.
"We have been working closely with medical experts and health authorities, and we strongly support the open communication of this information to healthcare professionals and the public," said Johson & Johnson.
According to Gudrun Briat of Belgium's Vaccination Taskforce, the decision will not affect the country's vaccination campaign in the short term.
"No deliveries of the Johnson & Johnson vaccine were expected in the next two weeks," she told the Belga news agency in a reaction. "Unless the entire vaccination with Johnson & Johnson is stopped, there is a good chance that we will not notice this delay."
At the press conference on Tuesday morning, however, Briat said that the new Johnson & Johnson vaccines would arrive in the vaccination centres this week, which would allow Belgium to shift its campaign into a higher gear.
On 11 March, the Johnson & Johnson vaccine was the fourth Covid-19 vaccine to receive the green light from the European Medicines Agency (EMA), which was expected as an important step for the vaccination campaigns in the EU, as the vaccine only requires a single dose.
Maïthé Chini
The Brussels Times