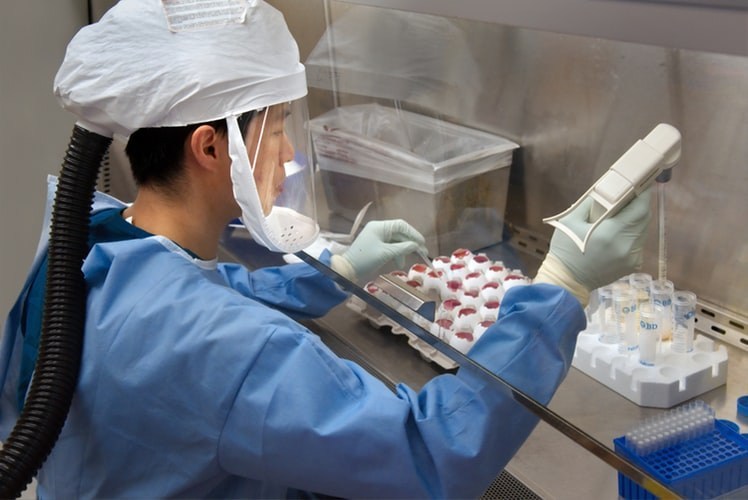
Belgium's Federal Agency for Medicines and Health Products (FAMHP) has already authorised 18 clinical trials involving treatments and vaccines against Covid-19, as of 18 June.
The trials are often collaborations between universities, pharmaceutical companies and hospitals, the agency announced on Tuesday.
Among these trials, one will test a candidate vaccine from the German biotech company CureVac.
In others, existing drugs that have already been approved for the treatment of other conditions are being tested to see if they could also be useful against coronavirus. These include the anti-malarial drugs chloroquine and hydroxychloroquine, the combination lopinavir/ritonavir, which treats and prevents HIV, and zilucoplan, which combats myasthenia gravis, a rare autoimmune disease.
Related News
- WHO calls for ramped-up production of 'life-saving drug'
- First coronavirus vaccine clinical trials to begin in Belgium
- Life-saving coronavirus drug 'promising' for Belgium, state virologist says
Eleven amendments were also adopted, allowing changes in the design of a previous clinical trial. These can be fairly drastic changes, such as testing another drug. "In this period of crisis, this makes it possible to react quickly," the agency pointed out.
All the trials are ongoing and could involve a total of up to 3,000 patients, who have all experienced significant symptoms, at 31 locations.
According to the FAMHP, 18 clinical trials and 11 amendments in such a short period of time for one disease is a lot. Every year in Belgium some 500 clinical trials are carried out by universities, pharmaceutical companies and hospitals.
The Brussels Times